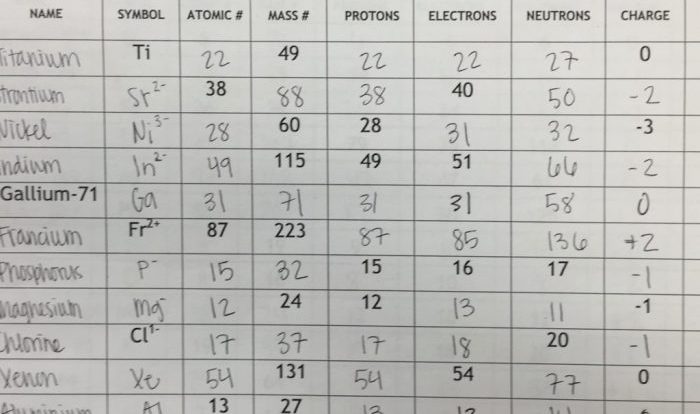
Predict the relative solubility of the following substances in water – Predicting the relative solubility of substances in water is a crucial aspect of chemistry, with applications in diverse fields such as drug delivery, chemical reactions, and environmental processes. This guide provides a comprehensive overview of the concept of solubility, factors affecting it, methods for predicting solubility, and practical applications of these predictions.
Solubility, the extent to which a substance dissolves in a solvent, is influenced by factors such as polarity, molecular size, and temperature. Common methods used to predict solubility include consulting solubility tables or databases, analyzing the chemical structure of the substance, and conducting experimental tests.
1. Introduction
Solubility refers to the ability of a substance to dissolve in a solvent. Water is a universal solvent, meaning it can dissolve a wide range of substances. The solubility of a substance in water is influenced by several factors, including its polarity, molecular size, and temperature.
Polarity measures the uneven distribution of electrical charge within a molecule. Substances with polar molecules tend to be more soluble in water because they can form hydrogen bonds with water molecules. Molecular size also plays a role, with smaller molecules generally being more soluble than larger molecules.
Temperature can also affect solubility, with higher temperatures generally increasing solubility.
2. Methods for Predicting Solubility
There are several common methods used to predict the relative solubility of substances in water:
- Solubility tables or databases:These resources provide experimental data on the solubility of various substances in water at different temperatures.
- Chemical structure:The chemical structure of a substance can provide clues about its solubility. For example, substances with polar functional groups, such as alcohols and acids, are typically more soluble in water than nonpolar substances, such as hydrocarbons.
- Experimental tests:Solubility can also be determined experimentally by measuring the amount of a substance that dissolves in a given volume of water at a specific temperature.
3. Examples of Substances with Varying Solubility
| Substance | Chemical Structure | Polarity | Molecular Size | Solubility in Water |
|---|---|---|---|---|
| Sodium chloride | NaCl | Polar | Small | Very soluble |
| Sugar | C12H22O11 | Polar | Large | Moderately soluble |
| Oil | CH3(CH2)16CH3 | Nonpolar | Large | Insoluble |
| Helium gas | He | Nonpolar | Small | Insoluble |
Sodium chlorideis a polar compound with a small molecular size, making it highly soluble in water. Sugaris also polar, but its larger molecular size reduces its solubility compared to sodium chloride. Oiland helium gasare both nonpolar, and their large molecular size and lack of polarity make them insoluble in water.
4. Applications of Solubility Predictions: Predict The Relative Solubility Of The Following Substances In Water
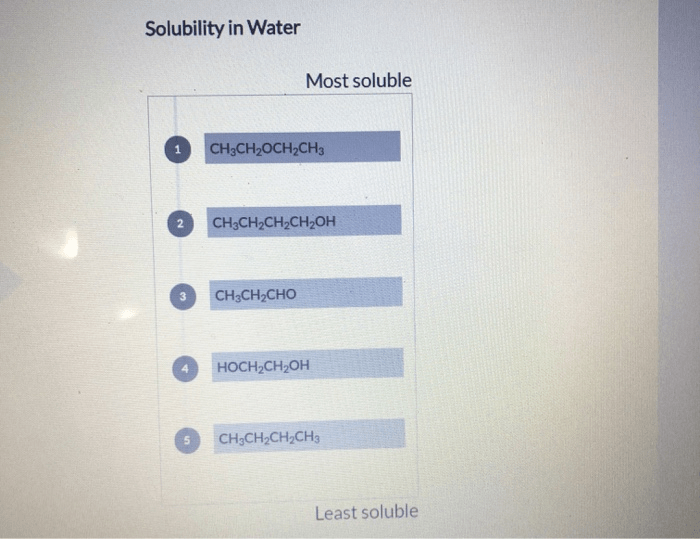
Predicting solubility has practical applications in various fields:
- Drug delivery systems:Solubility is a crucial factor in designing drug delivery systems, as it affects the bioavailability and efficacy of drugs.
- Chemical reactions:Solubility can influence the rate and extent of chemical reactions. For example, in aqueous solutions, only the dissolved reactants can participate in reactions.
- Environmental processes:Solubility plays a role in understanding the fate and transport of pollutants in aquatic environments.
FAQ Corner
What is the most important factor affecting the solubility of a substance in water?
Polarity is the most important factor affecting the solubility of a substance in water. Polar substances, which have a separation of charge, tend to be more soluble in water than nonpolar substances.
How can I predict the solubility of a substance in water?
There are several methods to predict the solubility of a substance in water. These include consulting solubility tables or databases, considering the chemical structure of the substance, and conducting experimental tests.