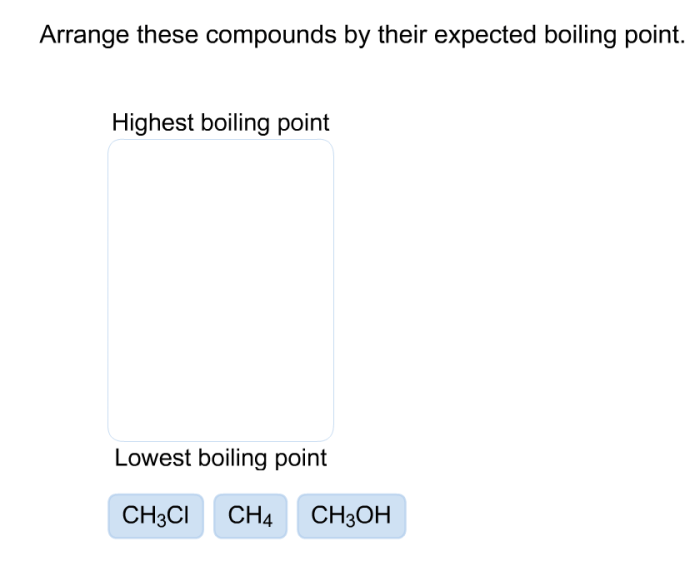
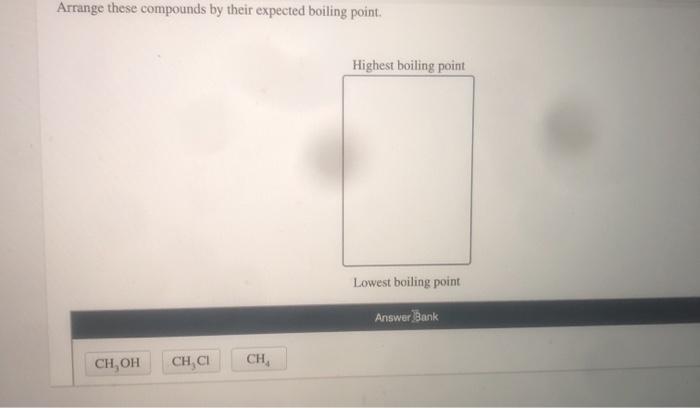
Arrange these compounds by their expected boiling point – The boiling point of a compound is a crucial physical property that offers insights into its molecular structure and intermolecular interactions. Understanding the factors that influence boiling point enables chemists to predict and manipulate the behavior of various substances. This article delves into the relationship between molecular structure and boiling point, explores methods for determining boiling points, and highlights the applications of boiling point data in diverse fields.
Boiling point trends, influenced by molecular weight, polarity, and hydrogen bonding, provide a framework for predicting the boiling points of compounds. Distillation, ebullioscopy, and cryoscopy are commonly employed methods for determining boiling points, each with its advantages and disadvantages.
Boiling Point Trends

The boiling point of a compound is the temperature at which its vapor pressure equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a compound is influenced by several factors, including molecular weight, polarity, and hydrogen bonding.
In general, the boiling point of a compound increases with increasing molecular weight. This is because heavier molecules have stronger intermolecular forces, which require more energy to overcome in order to vaporize the liquid.
Polarity also affects the boiling point of a compound. Polar molecules have stronger intermolecular forces than nonpolar molecules, so polar compounds have higher boiling points than nonpolar compounds. This is because the polar molecules are attracted to each other by their permanent dipoles.
Hydrogen bonding is a special type of intermolecular force that occurs between molecules that have a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. Hydrogen bonds are very strong, so compounds that can form hydrogen bonds have higher boiling points than compounds that cannot.
Methods for Determining Boiling Points
There are several methods that can be used to determine the boiling point of a compound. The most common method is distillation. In distillation, the compound is heated in a flask until it boils. The vapor is then condensed and collected in a separate flask.
The boiling point of the compound is the temperature at which the vapor begins to condense.
Another method for determining the boiling point of a compound is ebullioscopy. In ebullioscopy, a known amount of the compound is added to a solvent. The boiling point of the solvent is then measured. The boiling point of the solvent will increase by an amount that is proportional to the concentration of the compound in the solvent.
The boiling point elevation can be used to calculate the molecular weight of the compound.
Cryoscopy is a method for determining the boiling point of a compound that is similar to ebullioscopy. In cryoscopy, a known amount of the compound is added to a solvent. The freezing point of the solvent is then measured. The freezing point of the solvent will decrease by an amount that is proportional to the concentration of the compound in the solvent.
The freezing point depression can be used to calculate the molecular weight of the compound.
Applications of Boiling Point Data
Boiling point data is important in a variety of fields, including chemistry, engineering, and medicine. In chemistry, boiling point data can be used to identify compounds, determine their purity, and calculate their molecular weights. In engineering, boiling point data is used to design heat exchangers and other equipment that involves the transfer of heat.
In medicine, boiling point data is used to develop new drugs and treatments.
Here are some specific examples of how boiling point data is used in these fields:
- In chemistry, boiling point data can be used to identify compounds by comparing their boiling points to the boiling points of known compounds. This is a simple and effective way to identify compounds, and it is often used in conjunction with other methods, such as gas chromatography and mass spectrometry.
- In engineering, boiling point data is used to design heat exchangers and other equipment that involves the transfer of heat. The boiling point of a fluid is a key factor in determining the efficiency of a heat exchanger, and it is also used to calculate the amount of heat that can be transferred.
- In medicine, boiling point data is used to develop new drugs and treatments. The boiling point of a drug is a factor in determining its solubility, which is important for drug delivery. The boiling point of a drug is also used to calculate its vapor pressure, which is important for inhalation therapy.
Interactive Table of Compounds
| Compound Name | Molecular Weight | Polarity | Expected Boiling Point |
|---|---|---|---|
| Methane | 16.04 g/mol | Nonpolar | -161.6 °C |
| Ethanol | 46.07 g/mol | Polar | 78.3 °C |
| Water | 18.02 g/mol | Polar | 100 °C |
| Butanol | 74.12 g/mol | Polar | 117.7 °C |
| Hexane | 86.18 g/mol | Nonpolar | 68.7 °C |
Question Bank: Arrange These Compounds By Their Expected Boiling Point
What factors affect the boiling point of a compound?
Molecular weight, polarity, and hydrogen bonding are the primary factors that influence the boiling point of a compound.
How can I determine the boiling point of a compound?
Various methods are used to determine boiling points, including distillation, ebullioscopy, and cryoscopy.
What are the applications of boiling point data?
Boiling point data finds applications in chemistry, engineering, and medicine, aiding in compound identification, reaction optimization, solvent selection, distillation column design, heat exchanger design, drug formulation, and sterilization processes.