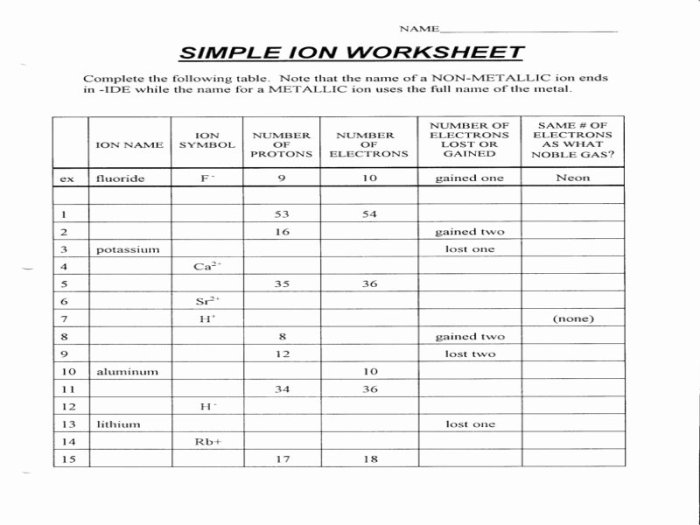
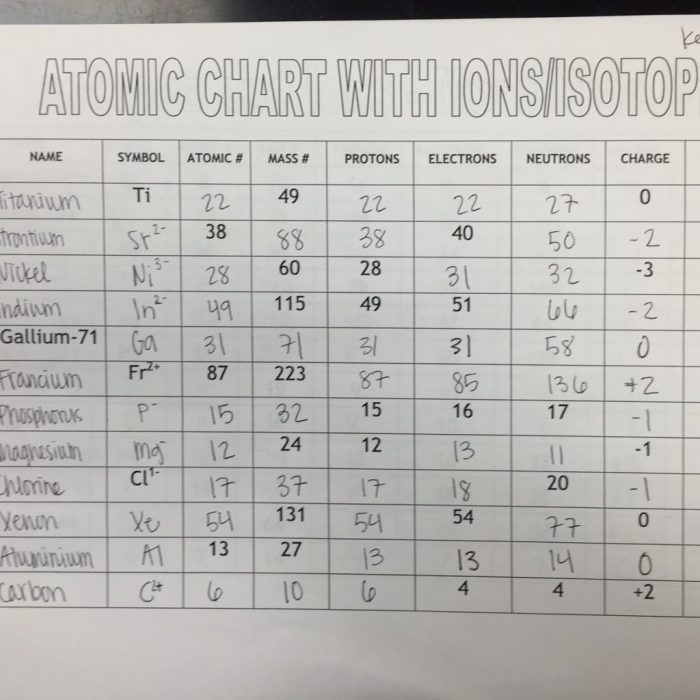
The Isotope and Ions Practice Worksheet provides a comprehensive exploration of the fundamental concepts and applications of isotopes and ions in chemistry. This worksheet delves into the intricacies of atomic structure, isotopes, ions, and their significance in various scientific fields.
Throughout this practice worksheet, students will engage with interactive exercises and thought-provoking questions designed to enhance their understanding of isotopes and ions. By completing this worksheet, students will gain a deeper appreciation for the role these particles play in chemistry and beyond.
Isotopes and Ions Overview: Isotope And Ions Practice Worksheet
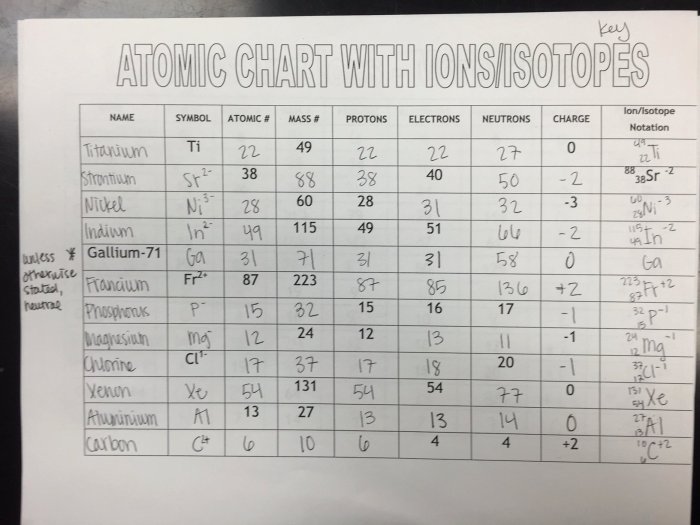
Isotopes and ions are fundamental concepts in chemistry that describe variations in atomic structure and charge. Understanding their differences and properties is crucial for comprehending chemical reactions and processes.
Isotopes
Isotopes are atoms of the same element that have the same atomic number but different neutron counts. This difference in neutron number results in different atomic masses for isotopes of the same element.
- Formation:Isotopes are formed through nuclear processes, such as radioactive decay or nuclear reactions.
- Examples:Carbon-12, carbon-13, and carbon-14 are isotopes of carbon with different neutron counts.
- Applications:Isotopes are used in various scientific fields, including dating techniques, tracing chemical reactions, and medical imaging.
Ions
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. The loss or gain of electrons alters the chemical properties of the atom or molecule.
- Formation:Ions are formed through chemical reactions, such as electron transfer or ionization.
- Types:Ions can be positively charged (cations) or negatively charged (anions).
- Properties:Ions have unique chemical and physical properties, such as solubility, reactivity, and electrical conductivity.
- Role:Ions play crucial roles in chemical reactions, biological processes, and electrochemistry.
Isotopes and Ions in Chemistry, Isotope and ions practice worksheet
Isotopes and ions are essential for understanding chemical reactions and processes. Isotopes contribute to the atomic mass of elements, and they can be used to trace chemical reactions and study reaction mechanisms.
- Atomic Mass:Isotopes contribute to the atomic mass of an element based on their relative abundance.
- Chemical Reactions:Isotopes can be used to study reaction mechanisms by tracing the movement of specific isotopes.
- Electrochemistry:Ions are the primary species involved in electrochemical reactions, such as battery operation and electrolysis.
- Solution Chemistry:Ions play a crucial role in solution chemistry, affecting properties such as conductivity, pH, and solubility.
Questions and Answers
What are the key differences between isotopes and ions?
Isotopes are atoms of the same element with different numbers of neutrons, while ions are atoms or molecules that have lost or gained electrons, resulting in a net electrical charge.
How are isotopes used in scientific research?
Isotopes are used in a variety of scientific fields, including medicine, archaeology, and environmental science, to trace chemical reactions, determine the age of materials, and study geological processes.
What are the different types of ions?
Ions can be classified as cations (positively charged) or anions (negatively charged). Cations are formed when atoms lose electrons, while anions are formed when atoms gain electrons.